🌏 The growth potential in international markets are significant but not an easy task. Especially for medical companies with little presence in international markets. More competition, pressure on hospital budgets, regulatory requirements, increasing labor costs while navigating complex fragmented markets and patient expectations – the numbers don’t lie, 70% of companies fail internationally. We’ve seen cases where medical companies rushed into Europe without really understanding how things work on the ground. Sometimes, language barriers made it tough to connect with potential customers or not having adequate local sales representation. Or missing the mark by not aligning prices or services with what the market expects. Key pitfalls:
1️⃣ 𝐈𝐧𝐚𝐝𝐞𝐪𝐮𝐚𝐭𝐞 𝐌𝐚𝐫𝐤𝐞𝐭 𝐑𝐞𝐬𝐞𝐚𝐫𝐜𝐡: A major reason why medical companies stumble abroad is insufficient market research. Failing to understand the unique needs, regulations, and competitive landscape in EU and MENAA markets can lead to a wastage of time and resources.
2️⃣ 𝐋𝐚𝐜𝐤 𝐨𝐟 𝐬𝐮𝐩𝐩𝐨𝐫𝐭𝐢𝐧𝐠 𝐬𝐚𝐥𝐞𝐬 𝐢𝐧𝐟𝐫𝐚𝐬𝐭𝐫𝐮𝐜𝐭𝐮𝐫𝐞: Managing sales from oversees will maintain operational complexities such as timezone, language barriers, cultural misalignment and inefficiencies of travelling resulting into ineffective representation, lower sales success and less effective engagement with local customers. Having local sales representation is a must for international sales success.
3️⃣ 𝐎𝐯𝐞𝐫𝐥𝐨𝐨𝐤𝐢𝐧𝐠 𝐑𝐞𝐠𝐮𝐥𝐚𝐭𝐨𝐫𝐲 𝐇𝐮𝐫𝐝𝐥𝐞𝐬: Navigating the labyrinth of regulations in different countries can be overwhelming. Companies often overlook the importance of complying with local laws and standards, leading to costly delays or product recalls.
4️⃣ 𝐏𝐫𝐨𝐝𝐮𝐜𝐭 / 𝐒𝐞𝐫𝐯𝐢𝐜𝐞 𝐚𝐝𝐚𝐩𝐭𝐚𝐭𝐢𝐨𝐧: Often companies do not realize the unique needs of local markets and the need for adaption that will require resources, flexibility and management commitment to make changes in the short term to gain long-term sales success.
5️⃣ 𝐑𝐞𝐬𝐨𝐮𝐫𝐜𝐞 𝐚𝐥𝐥𝐨𝐜𝐚𝐭𝐢𝐨𝐧: Companies that are successful in international markets have a long breathe and are investing in marketing, educational and building referral resources. Building a brand and becoming a trusted player in EU and MENA markets will require full long-term commitment.
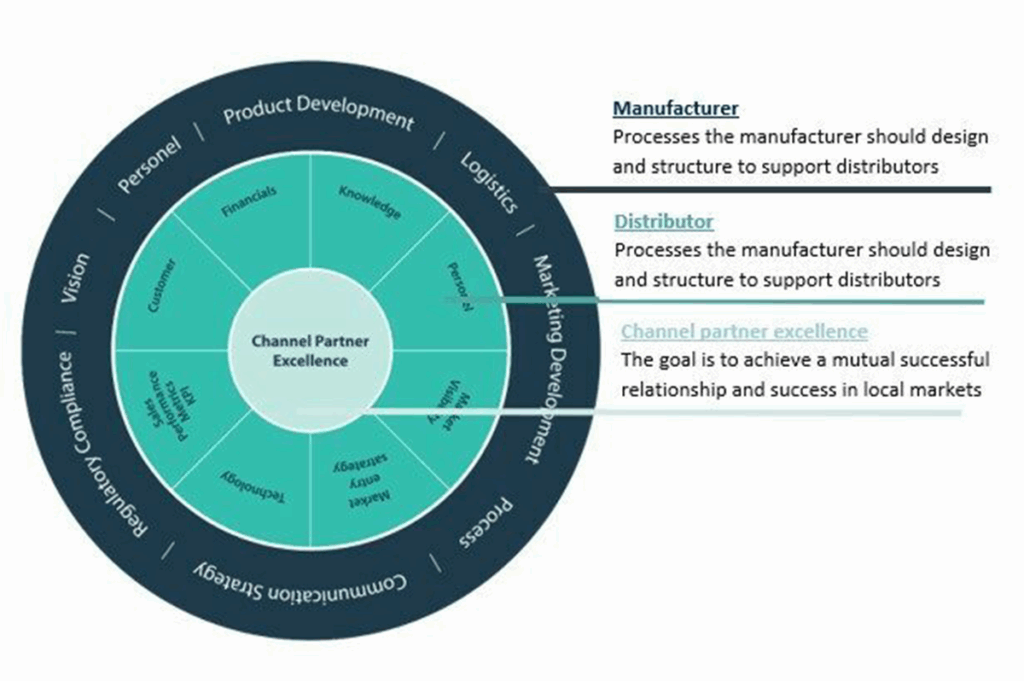
🎯 By joining forces with GrowthMedics, you’re tapping into a wealth of knowledge and local know-how that can help avoid these pitfalls.
📈 Through our in-country sales rep. services with access to 60+ healthcare markets and thousands of distributors, OEMs and hospitals and MDR/IVDR importing services we are dedicated to making worldwide medical companies successful in international markets.
📞 Let’s chat about your international opportunities.