Expanding into new markets, such as Europe or the Middle East, can be a significant opportunity for medical device manufacturers looking to grow their business. However, venturing into these regions brings a set of unique challenges, particularly in the realms of distribution and logistics. Selecting the right international medical device distributor is a critical decision that can impact your success in the new market.
In this article, we’ll explore the key factors to consider when choosing a distributor, and we’ll delve into the complex world of shipping, logistics, and supply chain issues associated with entering a new market.
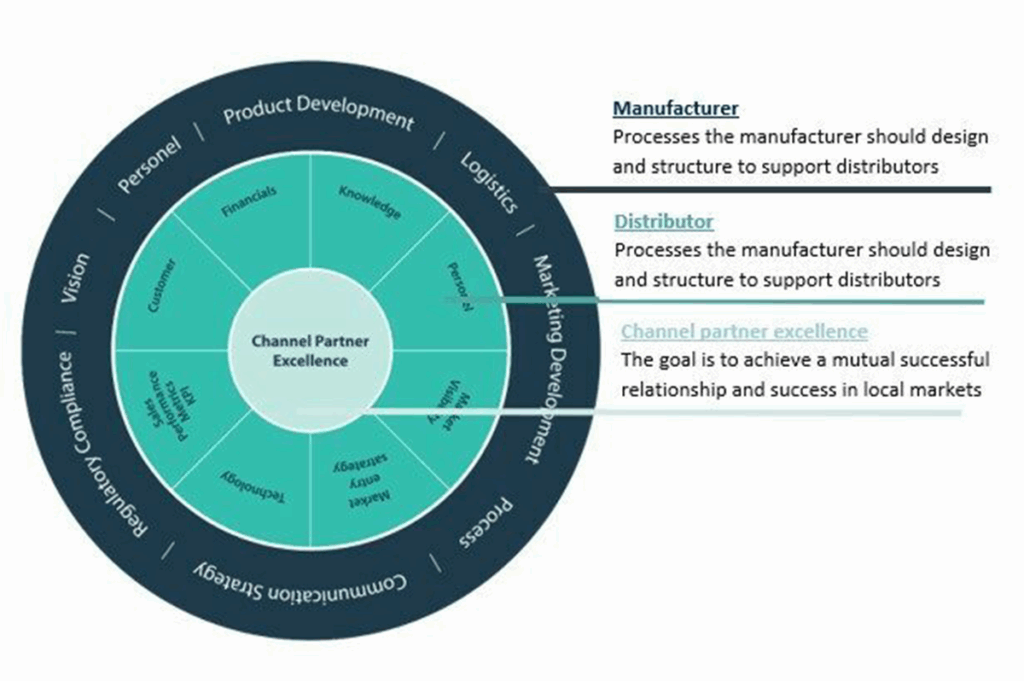
Selecting Your International Medical Device Distributors
Choosing the right distributor is paramount to your success in a new market. Here are some key factors to consider:
- Goals and Alignment: Define your goals and expectations clearly. Are you looking to increase market share, expand your customer base, or improve service quality? Ensure that your distributor aligns with these goals.
- Capabilities: Evaluate potential distributors’ capabilities thoroughly. Look for their experience, track record, and expertise in the medical device sector. Consider whether they have a dedicated sales team, an online presence, and an understanding of the local market.
- Market Understanding: A distributor who understands the local healthcare landscape, regulatory requirements, and customer preferences can provide valuable insights and help you tailor your products and marketing strategies effectively.
- Legal Agreements: Establish a clear legal framework for your partnership. Contracts should specify terms, expectations, responsibilities, and dispute resolution mechanisms. Legal experts well-versed in international trade can be invaluable.
- Capacity and Resources: Ensure your distributor has the resources and capacity to handle your product range efficiently. Consider their storage facilities, transportation infrastructure, and financial stability.
It may not always be possible to find a distributor that checks all the boxes, nor is it always the biggest distributor with all the resources that can bring you the success you desire. It is a consideration and trade-off that matches best with your capabilities and expectations.
If you have a new capital equipment product with long sales cycles a catalogue distributor may not be a good fit as big as they will be. If your products require more service and the distributor doesn’t have sufficient sales people, you may want to look for supportive lead generation to support the distributor with leads.
How hungry – motivated – is your distributor, how much do they believe in your product, how entrepreneurial and trustworthy are all questions that comes with the decision.
Addressing Shipping, Logistics, and Supply Chain Issues
Entering a new market introduces a host of supply chain complexities. Here’s what you should anticipate and address:
- Regulatory Compliance: Different markets have varying regulatory requirements for medical devices. Make sure your distributor is well-versed in local regulations and can assist in product registration, licensing, and compliance. The MDR / IVDR regulations require distributors to meet article 14. How well are distributors set up to meet these requirements.
- Import and Export Procedures: Navigating customs, duties, and import/export procedures can be daunting. Partner with a distributor who can efficiently handle these aspects to avoid delays and extra costs. Within the MDR/IVDR (article 13) it requires manufacturers to designate a regulatory importer. This can be a separate entity / economic operator. If the importer isn’t designated. Check out our regulatory importing services, www.growthimports.eu
- Product Labeling and Language: Ensure your products are appropriately labeled in the local language and conform to any required standards. This not only helps in regulatory compliance but also in improving customer understanding.
- Quality Assurance: Maintain a consistent level of quality control and assurance throughout the supply chain. Auditing and monitoring your distributor’s practices can be crucial in this regard.
- Stocking and Inventory Management: Establish a robust system for stocking and managing inventory. Demand forecasting, safety stock, and efficient order fulfillment processes are vital to avoid stockouts or overstocking.
- Transportation and Distribution: Select a distributor with a reliable transportation network to ensure timely deliveries. Evaluate their distribution strategies to optimize reach and minimize delivery lead times.
- Cold Chain Logistics: If your medical devices require temperature-controlled storage or transportation, assess your distributor’s capabilities in maintaining the cold chain effectively.
- Returns and Recalls: Develop clear protocols with your distributor for managing product returns and recalls, should they be necessary. Compliance with regulations and quality control is essential in these scenarios.
/*! elementor – v3.18.0 – 20-12-2023 */
.elementor-widget-image{text-align:center}.elementor-widget-image a{display:inline-block}.elementor-widget-image a img[src$=”.svg”]{width:48px}.elementor-widget-image img{vertical-align:middle;display:inline-block} 
Key Capabilities of a Distributor
To thrive in a new market, your distributor should possess specific capabilities:
- Sales Teams: A dedicated sales force with a deep understanding of the local market and your products can help in customer acquisition and retention.
- Online Presence: In the digital age, an online presence is vital. An e-commerce platform or an informative website can enhance visibility and accessibility.
- Marketing and Promotion: An effective marketing and promotion strategy tailored to the local market can give your products a competitive edge.
- Customer Service: Excellent customer service is crucial in healthcare. A distributor should be capable of providing timely support and addressing customer inquiries and concerns.
- Technical Support: For complex medical devices, technical support is essential. Ensure your distributor can handle maintenance and repair services efficiently.
Maximizing Efficiency When Expanding to a New Market With the Right Distributor is Key
Entering a new market in the medical device industry is a multifaceted endeavor, and selecting the right distributor is a pivotal step. Careful consideration of your goals, alignment with potential partners, and the capabilities they offer is crucial. Addressing the intricate web of shipping, logistics, and supply chain challenges is equally important.
In this journey, collaboration and communication with your distributor are key. Regularly assess the partnership, adapt to changes, and stay agile in response to evolving market conditions. With the right distributor and a well-thought-out strategy, expanding into new markets like Europe or the Middle East can be a rewarding endeavor for medical device manufacturers.
Get Started Today
GrowthMedics is an ISO 13485 market development provider specialized in assisting worldwide medical device manufacturers with their market expansion in European and Middle Eastern healthcare markets.
Having developed and managed hundreds of distribution partnerships, GrowthMedics, can support you in the process of finding and developing a successful distribution network.
Our sister company GrowthImports provides regulatory importing services in European and UK healthcare markets and can assist with selecting your international medical device distributor.
Email us or book a meeting with us. If you are interested in a free market scan you can apply here.