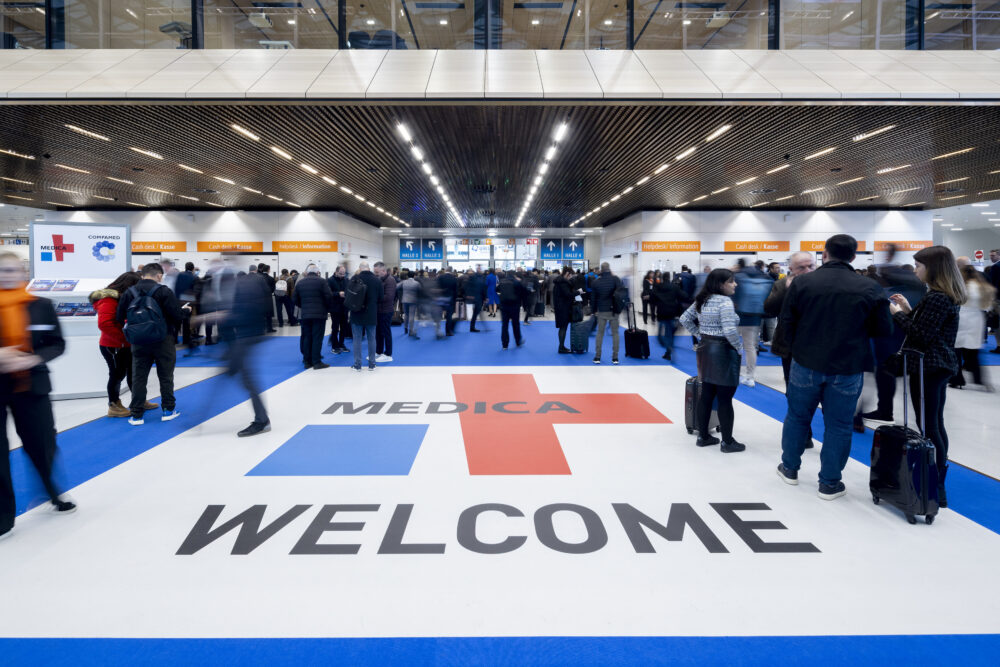
GrowthMedics is excited to see you all at Medica 2024 in Düsseldorf, which will be held from 11th to 14th November.
For over 40 years, MEDICA has been the top level tradeshow showcasing the latest innovations in medical technology, health care, and pharmaceuticals, drawing more than 5,000 exhibitors and tens of thousands of industry professionals from around the globe.
It is a perfect opportunity for your company to grow without any stress into the European healthcare market. With a proven track record across 60+ markets in Europe and MENA and access to thousands of hospitals, key opinion leaders (KOLs), distributors, and healthcare professionals in your industry, GrowthMedics could be the right partner to accelerate your growth and market expansion.
How we can help you:
- Increase your sales in the European and MENA markets
- Save significant cost on your marketing and sales efforts
- Instant access to 5000+ new qualified distributors, hospitals, OEMs, KOLs and healthcare professional in EU and MENA
- Install your dedicated sales office in Europe with sales support in 10+ languages
- Import your medical and IVD device compliant with the MDR/IVDR regulations
Striving for expansion? Please reach out to us or book call to explore the opportunities for your success!