HiCura Medical Pte Ltd is a MedTech company with the vision of empowering medical professionals by integrating Artificial Intelligence into image-guided procedures. The technology will now be offered for the international markets through a partnership with GrowthMedics.
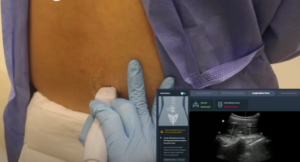
HiCura aims to enhance patient care by improving the accuracy and success rate of the first-attempt needle insertion during spinal injections with their patented AI-based imaging assistant – uSine It is an ultrasound guidance software that automatically identifies spinal landmarks during an ultrasound scan in real time, guiding the clinicians to the right needle insertion spot and angle, so they can get it right at the first try.
uSINE software runs on a commercial tablet and can be easily connected to any existing ultrasound machines. uSINE gives clinicians the confidence to move away from the blind palpation method to a safer and more efficient ultrasound image-guided way of performing neuraxial procedures.
“It’s a great pleasure to contribute and become part of a great knowledge team and such an innovative upcoming medical technology. The promising technology has been listed on Forbes Asia 100 to watch and has proven in numerous studies to have a 92% first attempt success rate for patients with BMI (<30). The solutions have proven to be safe and effective and promotes a reduction of procedural time, better clinical outcomes and improving patient satisfaction. This promising technology will have a great impact on the safety of spinal needle insertion in the European market” Emre Aykac – Managing director of GrowthMedics.
“The product is going to be available in Europe by Q3 of 2023 and will enable a safer and higher efficacy for doctors performing spinal injections on their patient using uSine guidance software. We are glad to partner with GrowthMedics because of their experience, knowledge and track record in launching new and innovative medical products in Europe.” John Teo – CEO of Hicura Medical.
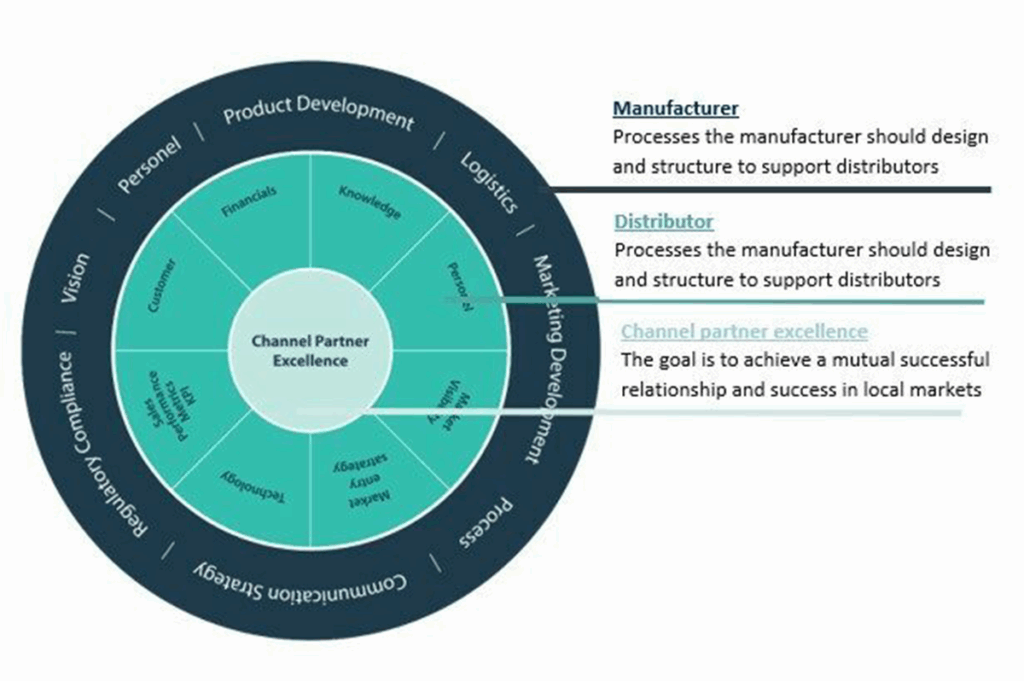
GrowthMedics’ team will secure a successful market introduction through the initial market assessment program. This will result into gathering feedback of key anaesthesiologists and key partners in the industry in selected European markets. By using our expertise and network we will be able to formulate a winning market entry strategy and execute to drive revenue.
European sales contacts and distributors
Hospitals and distributors in Europe interested in more information and a demonstration on location can contact HiCura Medical Europe’s European office:
HiCura Medical
Plesmanweg 9
7602 PD Almelo / The Netherlands
europe@hicuramedical.eu
About GrowthMedics
GrowthMedics is an ISO 13485 leading international market development provider making worldwide medical device companies successful in European and Middle Eastern healthcare markets.
GrowthImports is an ISO13485 regulatory and fiscal import partner in Europe and the United Kingdom for worldwide medical device manufacturers.