Learn more about the rise and structure of medtech clusters across selected European markets. Get access to a key supply chain to promote awareness and the development of new business opportunities for you as a manufacturer in the European market.
In today’s rapidly developing healthcare landscape, innovation is at the forefront of addressing complex medical challenges. These clusters are specialised in uniting medical device companies, research institutions, healthcare providers, and entrepreneurs in medical business to collaborate, innovate, and share the future of healthcare.
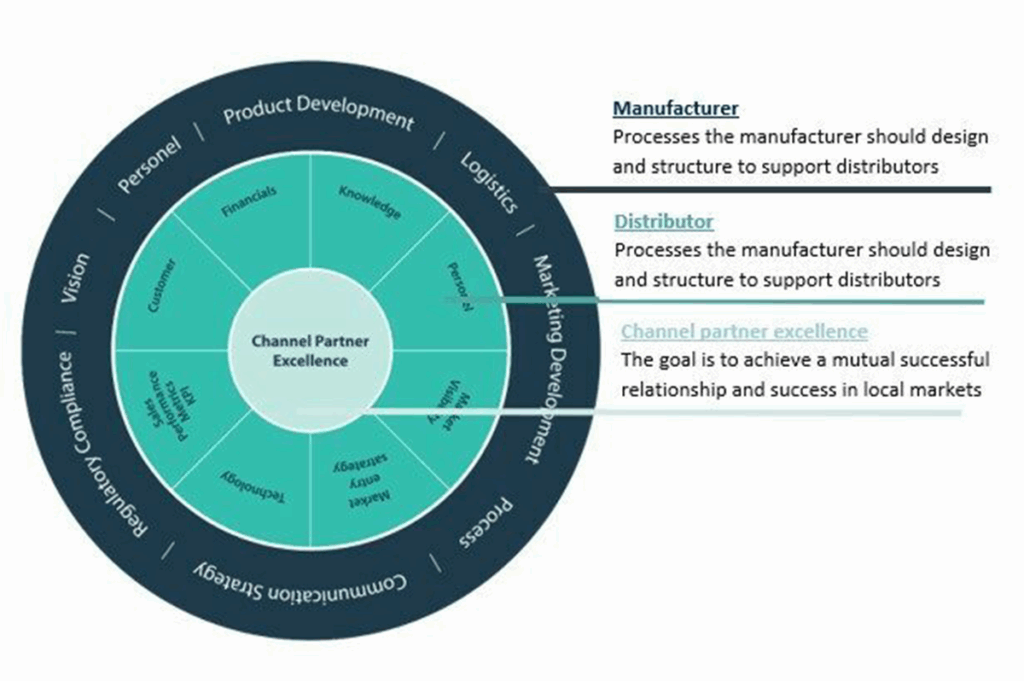
Europe is known for its innovations and high quality solutions aswell as being open for cooperations. The clusters will allow OEMs to identify and partner regionally with top innovative European medtech OEMs. It can provide the opportunity to become part of a key network that will give access to new partnerships such as universities, research organizations, funding and new business opportunities.
Here are some key points of why these medical clusters are important nowadays:
- Creating awareness and a professional brand for you as a manufacturer to partner with leading innovative medtech clusters
- Collaboration and Knowledge exchange
- Resource Sharing such as working with universities, funding and more
- Talent Pool
- Regulatory Expertise
- Investment Opportunities
- Increase Global Competitiveness by partnering with these clusters
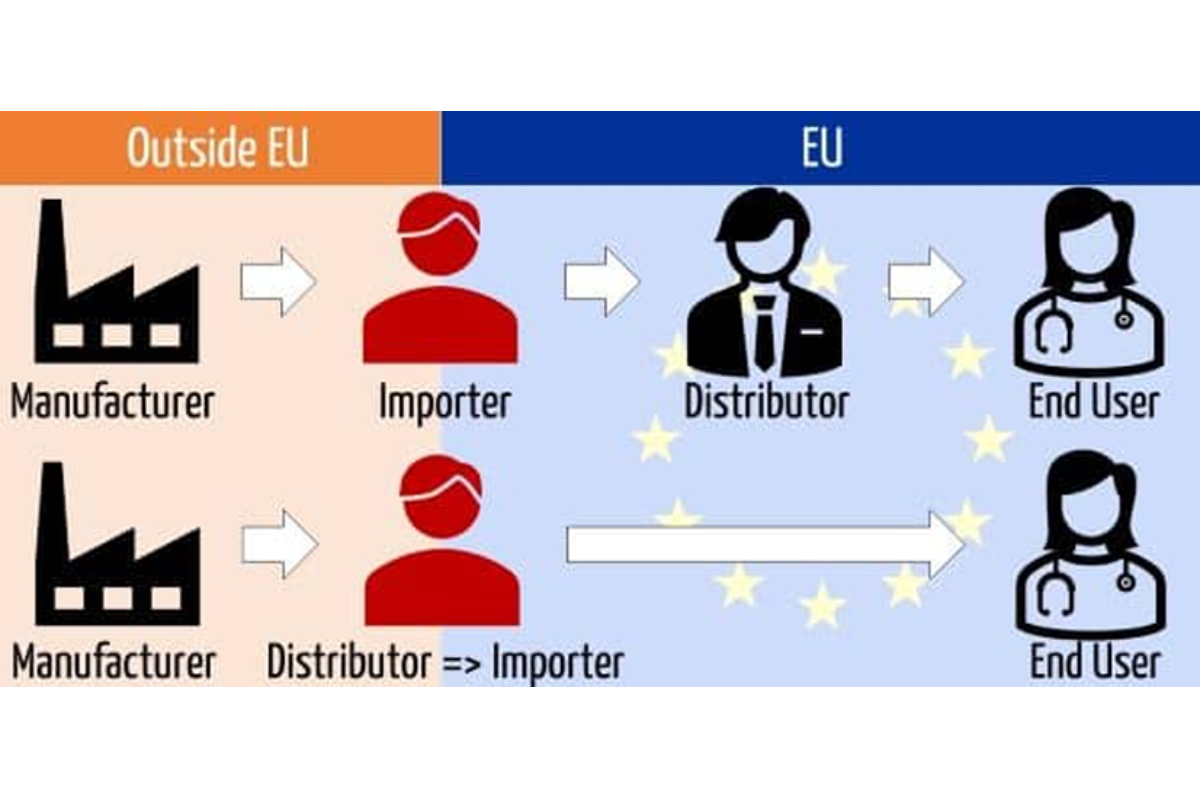
This overview provides insights in top European medtech clusters across selected EU countries. It contains information on its members, funding, supply chain and events.