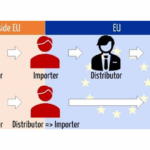
Learn how non-EU medical device manufacturers can stay MDR-compliant. This whitepaper explains what qualifies as an MDR importer, the risks of designating distributors, the legal obligations importers must meet under Article 13 and why choosing an independent EU-wide importer protects compliance, reduces risks, and maintains market control. You’ll learn how to map your supply chain, verify importer capabilities, ensure proper documentation, labeling, and traceability, and avoid costly compliance failures. The guide also highlights why relying on multiple distributors as importers can create liability risks and market complications. Finally, it introduces GrowthImports as an independent EU-wide MDR-compliant importer, offering cost-effective, flexible, and risk-free access to the European market while keeping manufacturers in full control. A must-read for companies looking to enter or expand in Europe safely and compliantly.