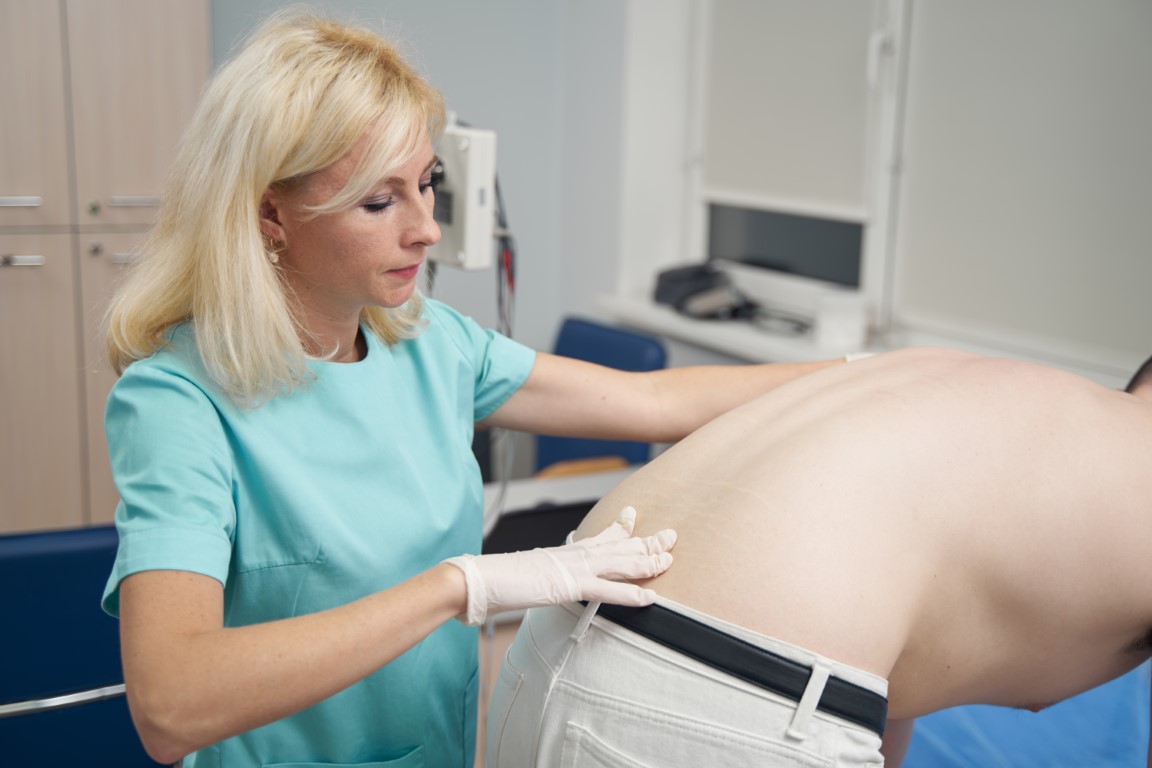
Orthopedic and Spine
Demand for orthopedic and spine products are growing rapidly due to the rising geriatric population in the world.
Home » Blogs and News » Page 2

Demand for orthopedic and spine products are growing rapidly due to the rising geriatric population in the world.
GrowthMedics is excited to see you all at Medica 2024 in Düsseldorf, which will be held from 11th to 14th
Duplicating market success in the European plastic surgery industry ClearPoint Medical has built a solid reputation as a manufacturer and

Proteomics International Laboratories Ltd (ASX: PIQ), a leader in predictive diagnostics, has engaged Growth Medics B.V. to expand the reach
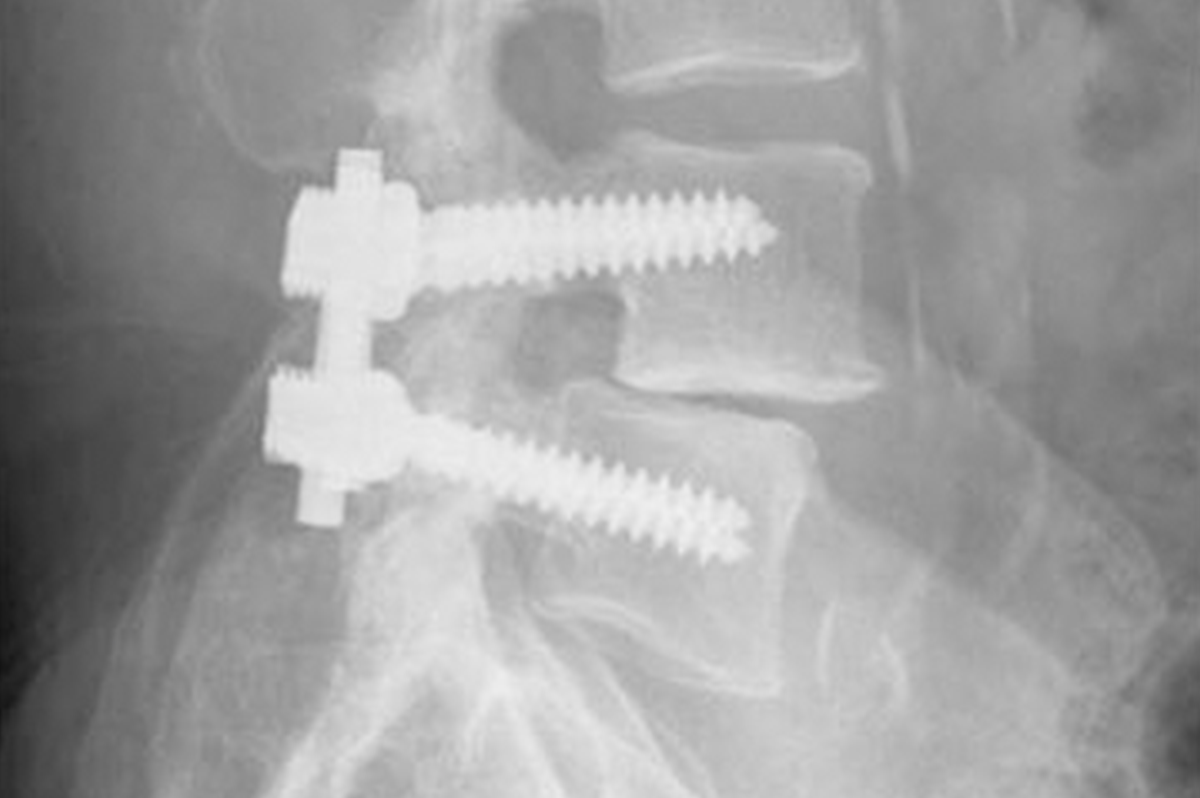
The European orthopedic and spinal market has shown steady growth over the past years and is forecasted to continue a

Germany is home to some of the most renowned medical device manufacturers in the world. A “made in Germany” stamp

The Spanish medical device (MED) market has grown to be worth €7.42 million, a number boosted by the government’s renewed

France is among the top 3 biggest spenders when it comes to healthcare expenditures in all of the EU. In

As the fourth largest medical equipment market in Europe, Italy’s high profit per capita rate seems inviting to investors and
Case Studies and Success Stories
Discover how a U.S. manufacturer expanded their distribution from 8 to 55 countries and quadrupled their revenue in Europe with the help of our medical device sales representatives. Read More.
In the ever-evolving landscape of the orthopedic and surgical industries, expanding into the European orthopaedic and spinal market offers immense opportunities alongside unique challenges. At
At GrowthMedics we specialize in supporting CMO’s with their expansion through European OEM markets. From injection molded solutions, coating services, to wearables, catethers, electronics and
Embarking on the journey to enter the European OEM market as a contract or component manufacturer in the medical industry? Careful planning and strategic considerations
Learn more about the rise and structure of medtech clusters across selected European markets. Get access to a key supply chain to promote awareness and
Per April 2023 Canadian medical manufacturers can apply for a funding for fiscal year 2023 and 2024! Don’t miss out on this opportunity and read
You may or may not have had your annual sales meetings with your distributors, if you haven’t it’s a critical moment to set out plans,
Breaking into Europe’s IVD market means navigating reimbursement, labs, and distributors —discover how GrowthMedics launched the world’s first predictive kidney test in Europe. The Challenge
Breaking into Europe with disruptive MedTech isn’t easy. Learn how GrowthMedics turned a 3-month pilot into a pipeline of OEMs, distributors, and hospital trials. The
European Anesthesia Market Overview The European medical technology market as a whole is enormous – about €170 billion in 2024, making Europe the world’s second-largest
A Growing Market Fuelled by Demand The European catheter market is experiencing steady and robust growth. This is largely due to an aging population, a
European IV Market Overview IV access products are a cornerstone of modern healthcare, as intravenous therapy is used daily across hospitals and clinics for hydration,
The European MedTech market is one of the most dynamic and competitive in the world. Yet, navigating this fragmented landscape of regulations, cultures, and stakeholders
Digital marketing is no longer just an option but an essential blueprint for any business looking to succeed in this modern environment. It’s not just
At GrowthMedics, we are proud to support global veterinary device manufacturers in establishing a robust presence in the dynamic European veterinary healthcare market. By leveraging
Whitepaper
Learn how non-EU medical device manufacturers can stay MDR-compliant. This whitepaper explains what qualifies as an MDR importer, the risks of designating distributors, the legal
The new EU MDR/IVDR regulations redefine the responsibilities of manufacturers, importers, and distributors—creating new risks for non-EU companies. This whitepaper explains why appointing your distributor
Europe/MENA quickly—bringing market know-how, KOL/distributor/hospital networks, and a proven outreach engine—while keeping costs, risk, and commitments low. You’ll compare SDaaS against hiring employees, independent reps,
Sucess Stories
From 14 to 55 distributors and 3.5x revenue growth in 3 years — discover how GrowthMedics helped a U.S. healthcare manufacturer scale across Europe. The
From zero presence to six exclusive distributors in 12 months—see how GrowthMedics launched a new veterinary rehab technology across Europe and the Middle East. The
When your domestic market matures, or if you are looking to scale revenue, growth means going global. Learn how GrowthMedics guided a U.S. infection control