At GrowthMedics we specialize in supporting CMO’s with their expansion through European OEM markets.
From injection molded solutions, coating services, to wearables, catethers, electronics and plastics. As new technologies and market trends arise in Europe, this blogs will give a brief overview on the European market.
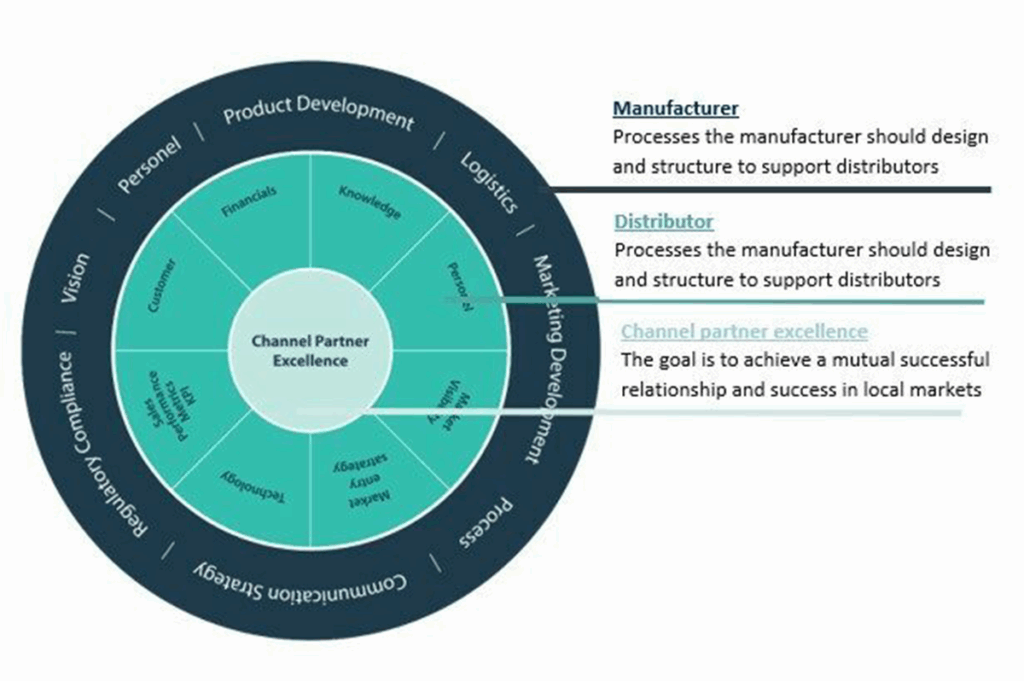
Check out our market expertise page on component manufacturing to read case studies and how we assist worldwide CMO’s gain new OEM partnerships across Europe.
Market size & growth:
Europe stands as a powerhouse in the global contract and component manufacturing landscape. According to recent market analyses, the European market has experienced significant growth in recent years, driven by factors such as advancements in technology, increasing demand for customized medical devices, and a robust regulatory framework ensuring product quality.
The market will grow at a CAGR of 6.0% during the forecast period of 2023 to 2030, reflecting a steady upward trajectory. This growth is indicative of the region’s prowess in providing high-quality manufacturing solutions that cater to the ever-evolving needs of the healthcare industry.
Current market trends in the industry:
Digitalization and Industry 4.0 Integration: The European market is witnessing a shift towards digitalization and Industry 4.0 practices. Smart manufacturing, data analytics, and automation are becoming integral components of the manufacturing process, enhancing efficiency and precision.
Focus on Sustainable Practices: Sustainability is no longer just a buzzword; it’s a driving force in manufacturing. European companies are increasingly adopting eco-friendly practices, aligning with the global movement towards environmentally responsible manufacturing.
Rise of Additive Manufacturing: 3D printing and additive manufacturing technologies are gaining prominence in the European market. These technologies offer unparalleled flexibility in design and rapid prototyping, revolutionizing the production of intricate medical components.
Largest OEM Markets in the European Medical Industry:
Germany: As previously highlighted, Germany leads the pack as the largest OEM market in Europe. German OEMs in the medical sector often specialize in producing high-tech components and devices, ranging from diagnostic equipment to surgical instruments. The focus is not only on meeting international standards but also on driving innovation and staying at the forefront of technological advancements.
This commitment positions German OEMs as key players in the global medical manufacturing landscape. While many are spread throughout the country, certain regions stand out as hubs for medical manufacturing. Southern Germany, including cities like Stuttgart and Munich, is known for its concentration of high-tech industries and manufacturing excellence. The Rhine-Main area, with Frankfurt at its core, is another hub, hosting a cluster of companies engaged in medical technology.
Switzerland: Renowned for its precision engineering and a strong emphasis on research and development, Switzerland is home to several influential OEMs in the medical industry. The Swiss OEM market is recognized for its contribution to high-tech medical devices.
United Kingdom: The UK boasts a robust medical manufacturing sector with a significant number of OEMs. The country’s OEMs play a vital role in producing a wide range of medical equipment, including diagnostic devices and surgical instruments.
France: With a rich history in medical research and development, France’s OEM market is marked by its focus on innovation. French OEMs contribute to advancements in medical imaging, laboratory equipment, and more.
Ireland: The rapid growth of contract and component manufacturers in Ireland is attributed to various factors. Ireland’s strategic location, within close proximity to both European and North American markets, facilitates efficient trade and logistics. Furthermore, the country’s investment in research and development, coupled with a robust regulatory framework, ensures the production of high-quality medical devices that adhere to international standards.
Italy: Several factors contribute to the rapid growth of contract and component manufacturers in Italy’s medical sector. Italy boasts a rich tradition of precision engineering, making it an ideal hub for producing high-quality medical equipment. This, coupled with a skilled workforce and advanced manufacturing technologies, positions the country as a key player in the global supply chain for medical devices.
Interested to learn how we helped a contract manufacturer expand in the European market through leading OEMs? Click here to read the case study.
If you are manufacturing components or are a contract manufacturing with the ambitions to enter and become a key player in the European medical OEM industry, feel free to reach out to us for a consultation.
Book a meeting with us here to meet at the MD&M West 2024!