Accessing the Swiss medical device market with FDA approval
The swiss medtech approves medical device manufacturers enter the Swiss healthcare market with FDA. This is good news for North American and worldwide medical device manufacturers with FDA and no CE mark or pending for MDR transition. They can now access the Swiss medical device market.
More than 1000 of the approximately 5000 foreign manufacturers have already stopped supplying Switzerland. They are not prepared to meet the requirements for the Swiss market.
The Swiss market has a global reputation for being outstanding. It combines public, subsidized private, and totally private healthcare systems to create an extensive network of highly qualified doctors and Swiss hospitals, the best equipped medical facilities, and no waiting lists. With high healthcare spendings the Swiss market offers opportunities for innovative premium medical devices.
Enter and expand the Swiss healthcare market
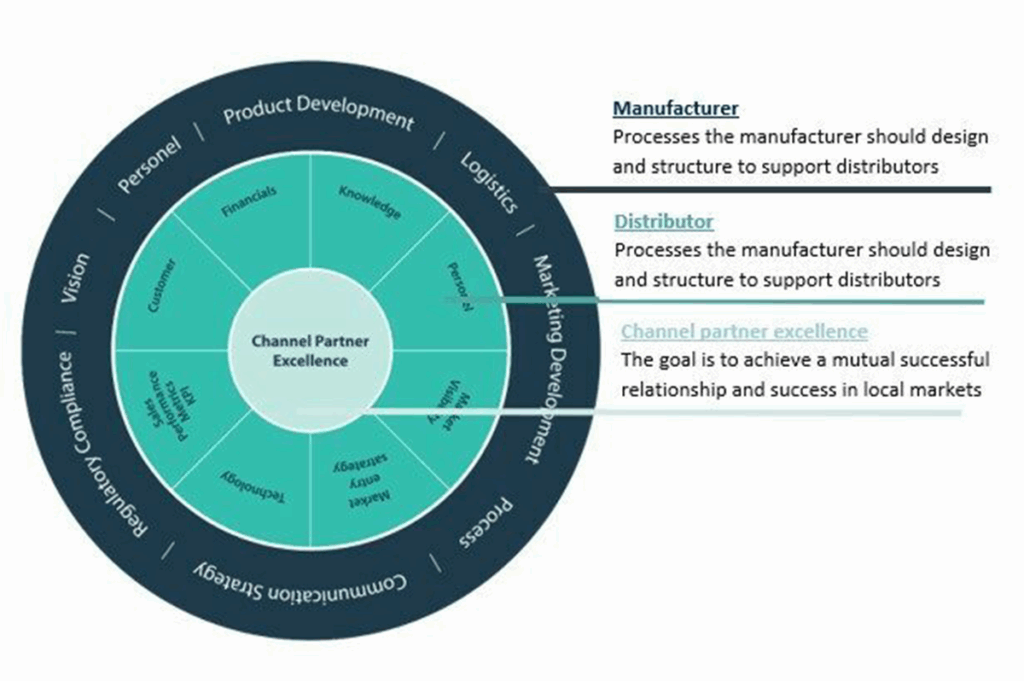
Our sales infrastructure has successfully assisted many medical device manufacturers with their expansion in the Swiss market. We have developed a special fast-track sales program to help medical device manufacturers expand through medical device distributors, OEMs and hospitals. The fast-track program aims to identify, develop and manage channel partners by utilizing our expertise and network.
Ask us how we can support you through our Swiss fast-track growth program.
Facts and figures about the Swiss market:
● There are 281 hospitals in Switzerland according to 2019.
● There are 4.59 beds per 1000 people in Switzerland
● 3.59 ICU Beds per 1000 people
●There are 154,000 employed nurses in Switzerland – this number is increasing every year.
● Density of medical doctors (per 10,000 population) (2012-2020): 43.8
● Density of nursing and midwifery personnel (per 10,000 population) (2012-2020): 182.6
● Density of dentists (per 10,000 population) (2012-2020): 4.1
● Density of pharmacists (per 10,000 population) (2012-2020): 6.7
● In Switzerland, almost 1.7 million people were aged 65 or older at the end of 2021
● In 2021 statistics showed 38,613 healthcare physicians employed in Switzerland
● Switzerland healthcare spending for 2019 was $9,666, a 2.07% decline from 2018.
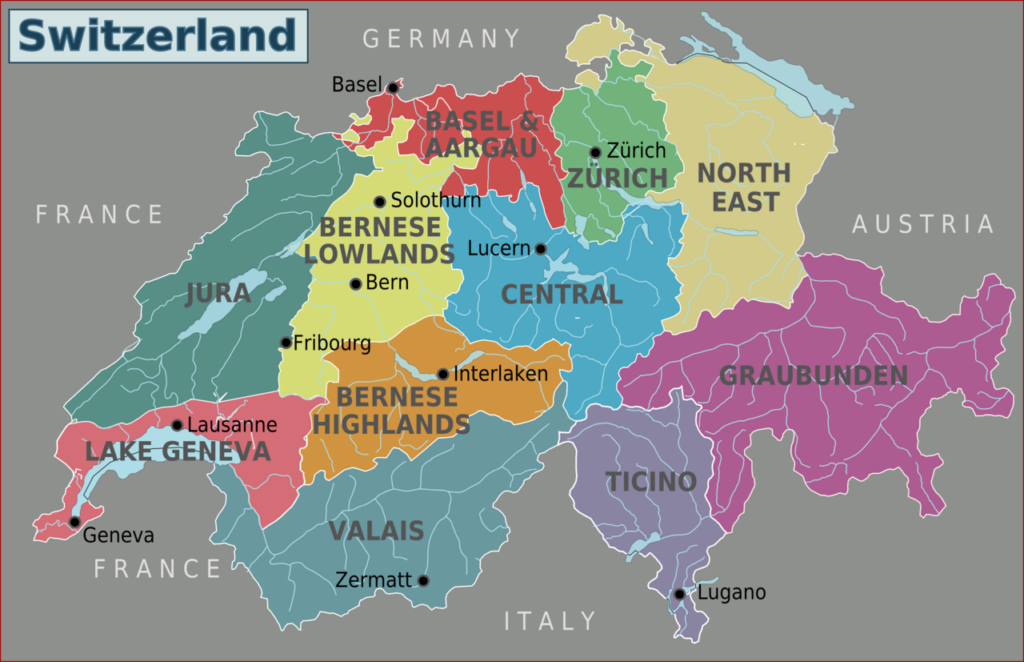
● 3 languages are spoken and divided by north & east (German), south (Italian) and west (French)
● The most populated regions is the north and north east part of the country
● The Swiss culture is distinguished by its diversity, as the country lies at the crossroads of several outstanding European cultures. Switzerland is also a multilingual country, as its national languages include German, French, Italian and Romansh. In addition, there are numerous dialects spoken in every region. Each canton has its unique cultural features. The culture, customs and traditions differ in different regions of the country, as each canton and municipality has cultural autonomy. 3 languages are mainly spoken and divided by north & east (German), south (Italian) and west (French)
● Switzerland is a financial and cultural center. It is housing enormous corporations, historical buildings, and a beautiful natural landscape. Due to its beneficial position in the heart of Europe and the fact of bordering four large European countries: Austria, France, Germany, and Italy, Switzerland is famous for multilingualism and multicultural environment.
● Switzerland engages in the trade of medical devices to the annual value of more than 16 billion Swiss francs, and this is showing an upward tendency. The value of the medical devices that are exported from Switzerland clearly exceeds the value of the imported medical devices. Switzerland has a positive trade balance with almost every country. Our most important trade partners are Europe, the USA and Asia. Devices from Swiss medical technology companies are in demand all over the world. Switzerland exports ,pre than 5,6Bn USD medical devices to the EU and Imports over 3Bn USD
If you have any questions or want to know more about expansion to the international markets – feel free to reach out to us or book a meeting with us here or contact us via contact@growthmedics.com