A Growing Market Fuelled by Demand
The European catheter market is experiencing steady and robust growth. This is largely due to an aging population, a rising number of chronic conditions, and the ongoing shift toward minimally invasive procedures. We project a CAGR of 7% between 2025 and 2030. We’ve noticed especially strong demand for specialized catheters in cardiology, neurology, and urology. Hospitals and clinics across Europe are actively seeking reliable, high-quality suppliers that can meet both patient needs and the strict EU regulatory standards.
The Path to Market Entry
For manufacturers outside of the EU, market entry involves navigating a few key challenges. The most significant are achieving MDR compliance and CE certification. From our experience, these regulations are complex and require a significant investment in time and resources. Beyond the red tape, success requires a tailored approach to pricing and a keen eye for finding trustworthy distributors. Local cultural nuances and procurement processes also vary greatly between countries, making local insights absolutely crucial for a smooth entry.
The Key to Long-Term Success
Simply having a great product isn’t enough to succeed. To stand out, companies must build strong relationships with their distributors, offer localized training and support, and ensure full compliance with all EU regulations. We believe that companies that demonstrate reliability, innovation, and adaptability are the ones that will truly thrive.
From a contract manufacturing (CMO) perspective, we’ve found that European companies are increasingly looking for partners who can offer specific, unique capabilities. For example, a manufacturer with a patented process for creating a more flexible catheter tip, or a company with a stellar track record in sterile packaging, can be an incredibly valuable partner. This is where a clear Unique Selling Proposition (USP) becomes a massive advantage. Companies will outsource to the US or other non-EU countries if they can find a partner with specific, specialized capabilities that would be too expensive or difficult to develop in-house.
Promising Markets to Target and Their Trends
While the entire continent offers opportunities, some markets are particularly attractive:
- Germany and France: As the largest markets with advanced healthcare infrastructures, they are essential targets. The trend here is consistent investment in cutting-edge technology and a focus on high-quality, reliable suppliers.
- The Netherlands and Belgium: These countries are known for being early adopters of new medical technologies. This makes them ideal for companies with truly innovative products.
- Poland and the Czech Republic: As emerging markets with growing demand and lower barriers to entry, they offer excellent opportunities for expansion. A key trend is the modernization of healthcare systems and a growing demand for cost-effective solutions.
The Future of the Catheter Market
The future of this market will be defined by miniaturization and specialization. We’ll see smaller, more flexible catheters that can reach more difficult areas of the body. There will also be a greater demand for smart catheters with built-in sensors for real-time monitoring and diagnostics. Furthermore, the push for sustainability will lead to the development of more environmentally friendly materials and production methods.
The catheter market is highly segmented, with each product group catering to a specific medical need. A look at the market share by product type shows the clear leaders. As the chart demonstrates, cardiovascular and urology catheters are the largest segments, but we are seeing significant growth and innovation in neurovascular and specialty catheters, which will play a major role in the future of minimally invasive procedures. Here is the approximate breakdown based on recent market data:
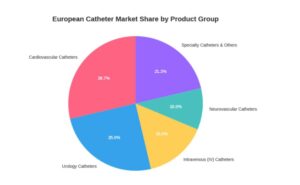
Figure 2 – European Catheter Market Share (Sergei Magdalena, 2025)
How GrowthMedics Can Help
At GrowthMedics, we specialize in helping non-EU catheter manufacturers enter the European market efficiently. We assist with everything from regulatory navigation to distributor selection and sales development strategies. By combining our local expertise with hands-on support, we help manufacturers reduce risks and accelerate their market success.